Guiding Principles
The first principle is that you must not fool yourself--and you are the easiest person to fool. So you have to be very careful about that. After you've not fooled yourself, it's easy not to fool other scientists. You just have to be honest in a conventional way after that.
— Prof. Richard Feynman
It doesn't matter how beautiful your theory is, it doesn't matter how smart you are. If it doesn't agree with experiment, it's wrong.
Core Research Areas
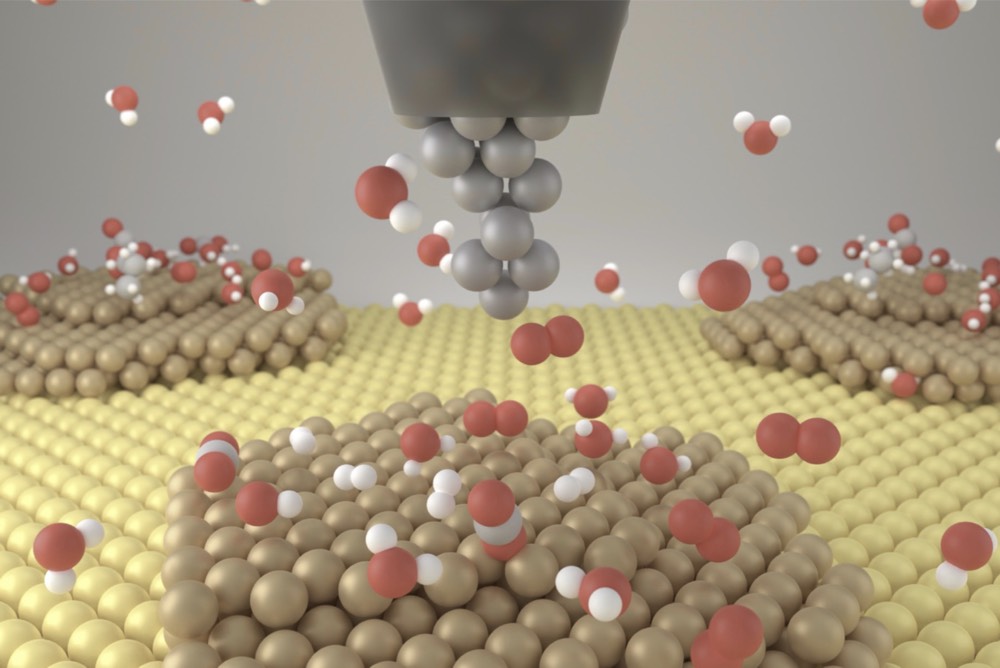
Operando Electrochemistry
The electrochemical interface is where the electron transfer between the electrode, electrocatalyst, and electrolyte. Understanding the interface reveals the structure-property relationship of electrocatalysts. In situ techniques are helpful to study the interface.
Our topic
- In situ electrochemical atomic force microscopy (EC-AFM)
- In situ differential electrochemical mass spectroscopy (DEMS)
Related publications
- J. Chen, et al., Journal of Catalysis, 2024, 438, 115720.
- W. Zheng, Chemistry Methods, 2023, 3(2), e202200042.
- W. Zheng, et al., ACS Energy Letters, 2021, 6, 2838–2843.
- W. Zheng, et al., ACS Catalysis, 2020, 10, 81–92.
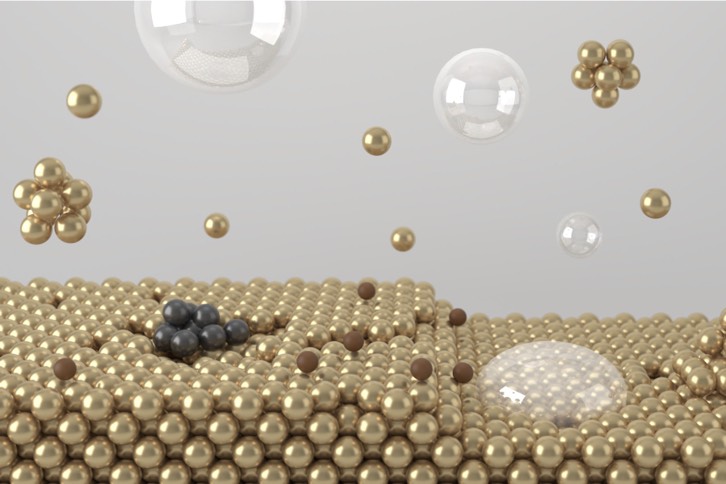
Electrocatalyst Design
Electrocatalysts are materials that can promote electrochemical conversion at a fixed potential. For the best efficiency, the materials must have a suitable surface atomic configuration and adsorption energy to achieve a good stability. The rational design of active sites is the best way to achieve this.
Our topic
- Electrochemical synthesis of inorganic materials
- Ammonia electrooxidation, water electrolysis
Related publications
- J. Zhong, et al., Electrochimica Acta, 2024, 497, 145178
- L. Du and W. Zheng, APL Energy, 2024, 2, 021501
- W. Zheng, Analysis & Sensing, 2023, 3(1), e202200070.
- W. Zheng, et al., Small, 2021, 17, 2007768.
- W. Zheng, et al., Nanoscale, 2021, 13, 15177–15187.
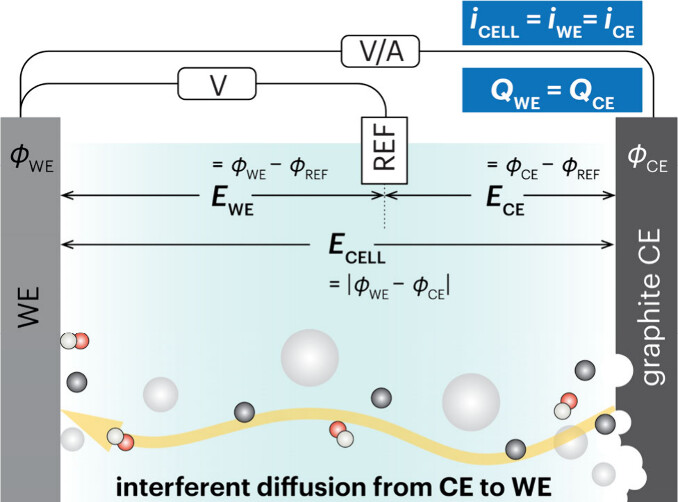
Electrochemistry Methods
Electrochemical results can be misleading if wrong concepts and inappropriate experimental procedures are adopted. Examining all details is essential to gaining a fundamental understanding of the electrochemical process.
Our topic
- Reproducibility of current electrochemical results
- Misleading concepts related to electrochemical process
Related publications
- W. Zheng and L. Du, ACS Energy Letters, 2024, 9, 4581-4586.
- W. Zheng, ACS Energy Letters, 2023, 8, 1952-1958.
- W. Zheng, et al., ACS Energy Letters, 2021, 6, 2838–2843.
- W. Zheng, et al., ACS Energy Letters, 2020, 5, 3260-3264.